In the hidden recesses of our nerve cells, approximately 3.7 billion individuals harbor the herpes simplex-1 virus, awaiting the moment it is awakened by stress or physical trauma. Once awakened, it tends to manifest itself in very mild forms, usually presenting itself as mouth ulcers or cold sores.
In extremely rare cases, the virus has the ability to migrate through neurons into the brain, resulting in a potentially fatal infection. This occurrence represents approximately 5 to 15% of all reported cases of infectious encephalitis in individuals of all ages. Typically, medical professionals will administer a prescription for the antiviral drug known as acyclovir. However, despite this treatment, patients often experience persistent and disabling symptoms such as memory impairment, seizures, and various cognitive impairments.
Scientists at the Max Delbrück Center for Molecular Medicine at the Helmholtz Association in Berlin propose that, in situations like these, medical professionals can improve the prognosis by administering a combination of an antiviral and an anti-inflammatory drug. This suggestion is based on a recent study published in Nature Microbiology. The researchers arrived at this conclusion using a three-dimensional brain model that was grown from human stem cells. These models, known as organoids, represent a cutting-edge approach in the field of clinical medicine.
Professor Nikolaus Rajewsky, scientific director of the Berlin Institute of Medical Systems Biology at the Max Delbrück Center (MDC-BIMSB) and senior author of the study, expressed that these early-stage brains have a myriad of neurons, numbering in the hundreds of thousands, that have the ability to communicate synchronously. This remarkable development allows the execution of crucial experiments that were inconceivable just a few years ago.
The creation of the organoids, measuring 0.5 cm and looking like white bubbles, was carried out by Dr. Agnieszka Rybak-Wolf, leader of the Organoid Technology Platform at the Max Delbrück Center and a key contributor. Describing the appearance of these brain organoids, she compares them to small clumps of tissue that resemble clouds.
Moving towards a more realistic representation of the herpes virus
Studying HSV-1-induced encephalitis becomes a difficult task in the absence of organoids. Given that the virus exclusively infects humans, acquiring brain samples for analysis is not a viable option. Therefore, the scientists chose to investigate the disease using cultured nerve cells or mice, as these animals are not natural hosts for the virus.
According with the doctor. Emanuel Wyler, a renowned virus researcher specializing in the study of HSV-1 infections at the Landthaler laboratory, the current model for the herpes virus is significantly more accurate than its predecessor. doctor Wyler, who is a major contributor to this research, focuses on unraveling the intricate molecular mechanisms underlying HSV-1 infections.
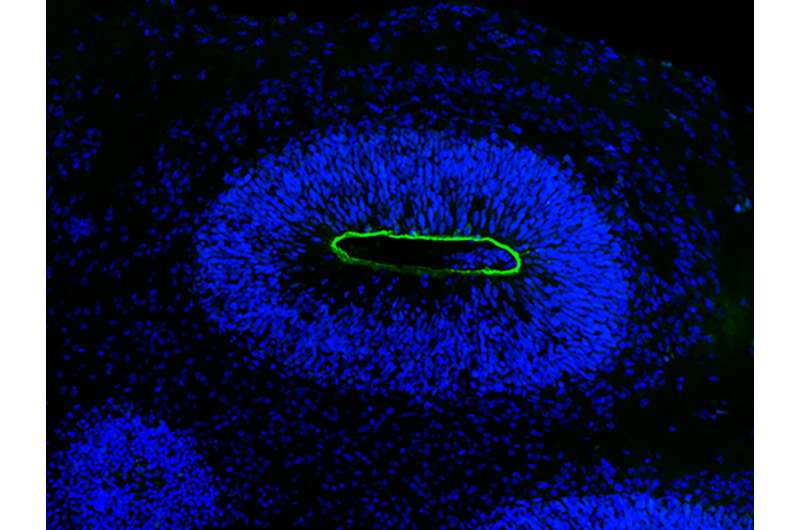
The researchers intentionally introduced the HSV-1 virus into organoids and closely observed the progression of the virus as it infiltrated neuroepithelial and neuronal cells, resulting in the gradual deterioration of the mini-brain structure. Wyler notes that the microscopy images captured during this process are exceptionally detailed, providing a vivid depiction of the events unfolding within them.
Next, they performed analysis at the level of individual cells to discern the full range of molecular pathways that are active during infection. “Our approach was completely unbiased, allowing us to identify all the pathways and genes that are important,” says Dr. Ivano Legnini, a systems biologist who was formerly associated with the Rajewsky lab and is a key contributor to the study. . “In essence, we are introducing the field of systems biology into this investigation.
Upon observation, it was clear that there was a significant level of activity in a signaling pathway, known as TNF-α, which plays a crucial role in inflammation. Later, when the organoids were treated with acyclovir, the standard drug for HSV-1 encephalitis, virus replication ceased, although the resulting tissue damage persisted.
Upon further examination, it was found that the TNF-α pathway remained active even after treatment.
A Defense Mechanism That Has the Potential to be Harmful
The Dr Tancredi Massimo Pentimalli, a medical professional currently pursuing his doctorate in systems medicine at the Rajewsky lab and one of the study’s lead authors, explains that the inflammation pathway serves as a crucial innate defense mechanism against the virus. However, when antiviral drugs are used to stop viral replication, the excessive inflammatory reaction can potentially lead to harmful effects.
To prevent any damage to the mini-brains, Rybak-Wolf administered an antiviral and anti-inflammatory treatment, effectively disabling the TNF-α pathway. According to Rybak-Wolf, the brain has a specific signaling pathway that becomes active in case of infection. By using these drugs to suppress this pathway, the organoids remained unharmed.
In the search for a viable treatment for HSV-1 encephalitis, scientists are recommending the use of acyclovir along with an anti-inflammatory drug. Pentimalli expresses his hope that clinicians will conduct clinical trials to evaluate the effectiveness of these new combination therapies in treating patients with herpetic encephalitis. The aim is to bridge the gap between laboratory discoveries and practical application in the medical field.
More information: Nikolaus Rajewsky et al, Modeling herpes simplex virus 1 infection in brain organoids reveals potential new therapeutic approaches for viral encephalitis, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01405-yhttps://dx.doi.org/10.1038/s41564-023-01405-y . www.nature.com/articles/s41564-023-01405-y
Journal information: Nature Microbiology